Release Date:2022-11-07 11:12:26 Popularity:
The virus sampling tube produced by GST nucleic acid detection virus sampling tube filling and capping packaging automatic production line equipment belongs to medical device products. Most domestic manufacturers are registered as first-class products, and few companies are registered as second-class products. For the urgent needs of Wuhan and other places, many enterprises are applying for the first-class filing license through the "emergency channel". The virus sampling tube is composed of sampling swab, virus preservation solution and outer packaging. Since there is no unified national standard or industry standard, the products of various manufacturers vary greatly.
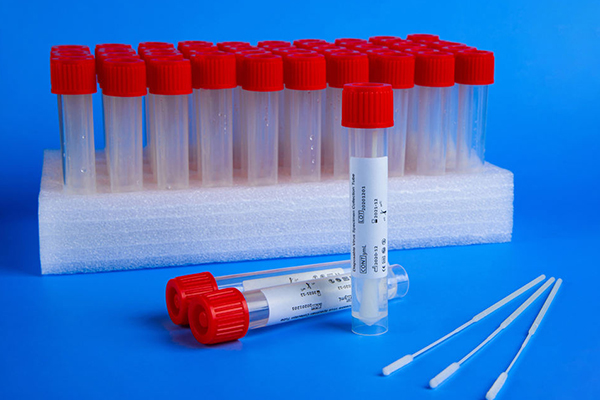
1. Sampling swab: The sampling swab directly contacts the sampling site, and the material of the sampling head is closely related to the subsequent detection. The sampling swab head should be made of Polyester (PE) synthetic fiber or Rayon (man-made fiber). Calcium alginate sponge or wooden stick swabs (including bamboo sticks) cannot be used, and the material of the swab head cannot be cotton. Because the fiber of cotton has strong adsorption of protein, it is not easy to be eluted into the subsequent preservation solution; and the wooden stick or bamboo stick containing calcium alginate and wooden components will also adsorb protein after being broken and soaked in the preservation solution, and even will It has an inhibitory effect on the subsequent PCR reaction. It is recommended to use synthetic fibers such as PE fiber, polyester fiber and polypropylene fiber for the material of the swab head. Natural fibers such as cotton are not recommended, nor nylon-based fibers, because nylon-based fibers (similar to toothbrush heads) are absorbent. Poor, resulting in insufficient sampling, affecting the detection rate. Calcium alginate sponge is prohibited for sampling swab materials! There are two types of swab handles: broken type and built-in type. The broken swab is placed in the storage tube after sampling, and then the tube cover is broken after being broken off near the sampling head; for the built-in swab, the sampling swab is directly placed into the storage tube after sampling, and the built-in cap of the storage tube is Align the small hole with the top of the handle and tighten the cap. Comparing the two methods, the latter is relatively safe. When a broken swab is used with a smaller storage tube, it may cause the liquid in the tube to splash out when broken, and full attention should be paid to the possible contamination risk caused by improper use of the product. The material for making the swab handle is recommended to use hollow polystyrene (PS) extrusion tube or polypropylene (PP) injection indentation tube, no matter what material is used, calcium alginate additives cannot be added; wooden sticks or Bamboo sticks. In short, the sampling swab should ensure the sampling amount and release amount, and the selected material must not contain substances that affect the subsequent detection.
2. Virus preservation solution: There are mainly two kinds of virus preservation solutions widely used in the market, one is a virus maintenance solution improved on the basis of transport medium, and the other is an improved preservation solution of nucleic acid extraction lysate.
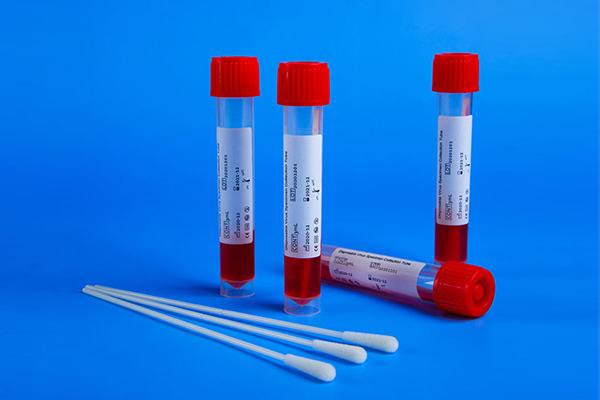
The main component of the former is Eagle's basal medium (MEM) or Hank's balanced salt, adding salts, amino acids, vitamins, glucose and proteins required for virus survival. This preservation solution uses phenol red sodium salt as an indicator. The solution is pink at pH 6.6-8.0. The necessary glucose, L-glutamine and protein are added to the preservation solution, and the protein is provided in the form of fetal bovine serum or bovine serum albumin, which can stabilize the protein coat of the virus. Since the preservation solution is rich in nutrients, it is conducive to the survival of viruses but also to the growth of bacteria. If the preservation solution is contaminated with bacteria, it will multiply, and the carbon dioxide in its metabolites will reduce the pH of the preservation solution. turns yellow. Therefore, most manufacturers have added bacteriostatic ingredients in the formula. The recommended bacteriostatic agents are penicillin, streptomycin, gentamicin and polymyxin B, etc. Sodium azide, 2-methyl methacrylate are not recommended. Inhibitors such as yl-4-isothiazolin-3-one (MCI) and 5-chloro-2-methyl-4-isothiazolin-3-one (CMCI), as these components have an effect on the PCR reaction. Since the samples provided by this preservation solution are basically live viruses, the originality of the samples can be maintained to the greatest extent, and it can be used not only for the nucleic acid extraction and detection of viruses, but also for the cultivation and isolation of viruses. However, when it is used for detection, it should be noted that nucleic acid extraction and purification must be carried out after inactivation.
Another preservation solution based on nucleic acid extraction lysis solution, the main components are balanced salts, EDTA chelating agent, guanidine salts (such as guanidine isothiocyanate, guanidine hydrochloride, etc.), anionic surfactants (such as dodecane) Sodium sulfate), cationic surfactants (such as tetradecyl trimethyl ammonium oxalate), phenol, 8-hydroxyquinoline, dithiothreitol (DTT), proteinase K and other several or more components, This preservation solution is to directly lyse the virus to release nucleic acid and eliminate RNase. If it is only used for RT-PCR, it is more suitable, but the lysate can inactivate the virus, and this sample cannot be used for virus culture and isolation.
EDTA salts (such as dipotassium ethylenediaminetetraacetate, disodium ethylenediaminetetraacetate, etc.) are recommended for metal ion chelators used in virus preservation solutions, and heparins (such as sodium heparin, lithium heparin) are not recommended to avoid affecting PCR detection.
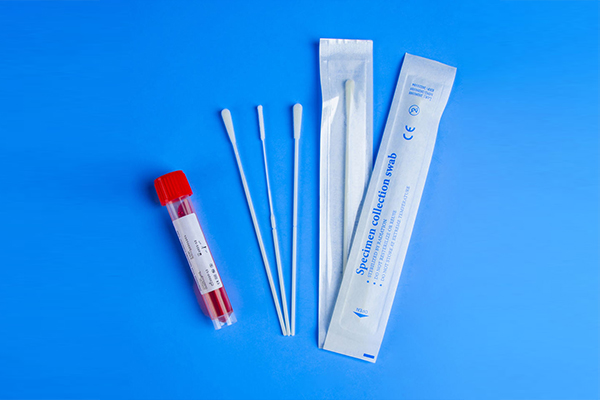
3. Preservation tube: The material of the preservation tube should be selected carefully. Some data suggest that polypropylene (Polypropylene) is related to the adsorption of nucleic acids, especially in the case of high tension ion concentration, polyethylene plastic (Polyethylene) is more than polypropylene (Polypropylene). Easy to grab DNA/RNA. Polyethylene-propylene polymer (Polyallomer) plastic and some specially treated polypropylene (Polypropylene) plastic containers are more suitable for DNA/RNA storage. In addition, when using breakable swabs, the storage tube should try to choose a container with a height of more than 8 cm to prevent the contents from splashing and contaminating when the swab is broken.
4. Water for the production of preservation solution: Ultrapure water used for the production of preservation solution should be filtered through an ultrafiltration membrane with a molecular weight of 13,000 to ensure the removal of biologically derived polymer impurities, such as RNase, DNase and endotoxin, and ordinary purification is not recommended. water or distilled water.